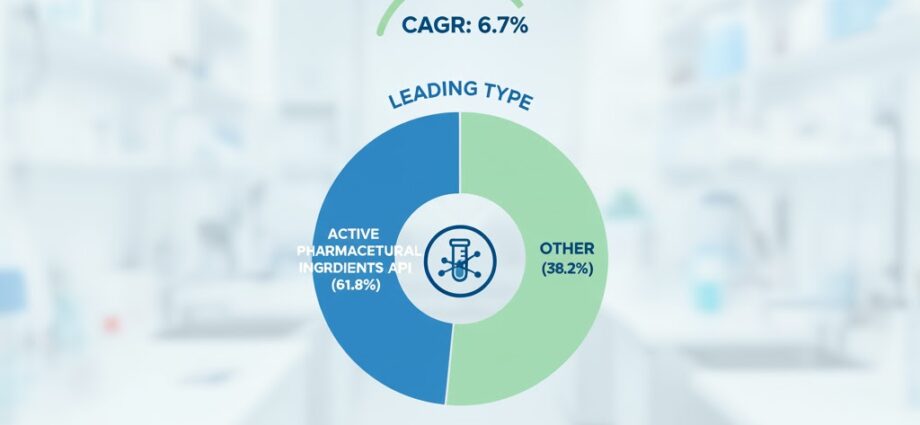
The Small Molecule CDMO Market is poised for remarkable growth, projected to rise from USD 76.1 billion in 2025 to USD 145.5 billion by 2035—an absolute increase of USD 69.4 billion, representing 91.2% total growth. The market is forecast to expand at a CAGR of 6.7%, driven by the escalating outsourcing of pharmaceutical manufacturing, demand for specialized capabilities, and emphasis on cost efficiency within the pharmaceutical value chain.
Explore trends before investing – request a sample report today!
Regional Growth Drivers: APAC Leads, Europe and USA Sustain Momentum
The Asia Pacific region (led by China and India) represents the fastest-growing market, supported by government initiatives and expanding pharmaceutical manufacturing capacity. China is anticipated to record a CAGR of 9.0%, while India follows at 8.4%, demonstrating strong capabilities in both generic and innovative manufacturing.
In Europe, Germany, France, and the UK collectively anchor growth with technical excellence and strong regulatory frameworks. Germany remains a central hub with 7.7% CAGR, driven by high-quality manufacturing and digital innovation. France’s specialized manufacturing capabilities and the UK’s thriving pharmaceutical ecosystem further reinforce Europe’s position in global CDMO expansion.
Meanwhile, the USA continues to lead in innovation and advanced chemistry expertise with 5.7% CAGR, maintaining its status as a global center for small molecule innovation and regulatory excellence. Saudi Arabia, supported by ongoing Vision 2030 healthcare initiatives, is emerging as a strategic growth hub for pharmaceutical manufacturing and CDMO partnerships across the Middle East.
Market Segmentation and Dominant Product Categories
By product outlook, Active Pharmaceutical Ingredients (APIs) account for 61.8% of the market in 2025, reflecting the pivotal role of complex chemistry and regulatory knowledge in CDMO operations. The Innovator drug segment holds a 55% market share, underscoring increasing collaboration between innovative pharma companies and CDMOs for rapid, compliant manufacturing solutions.
Oncology remains the largest therapeutic application, comprising 35% of total market share, driven by the expanding pipeline of targeted cancer therapies and demand for high-containment facilities.
Technological Advancements and Integration of End-to-End Solutions
Modern CDMOs are redefining operational excellence through continuous manufacturing, process analytical technologies, and digitalized workflow management. Continuous manufacturing has become a hallmark of quality and efficiency, enabling faster turnaround and consistent product integrity.
Integrated service portfolios spanning early-stage development to commercial manufacturing are transforming client engagement, offering single-source solutions that simplify technology transfer and ensure seamless scalability.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates.
Competitive Landscape and Market Positioning
The competitive environment is defined by innovation, consolidation, and the expansion of full-service capabilities. Lonza, Catalent Inc., and Thermo Fisher Scientific Inc. remain at the forefront of global leadership, offering comprehensive drug development and manufacturing services. Regional leaders such as Bellen Chemistry (China), Aurigene Pharmaceutical Services Ltd. (India), and Recipharm AB (Sweden) are scaling their offerings to meet global regulatory and quality benchmarks.
Emerging players in APAC and the Middle East are focusing on quality certifications, digital transformation, and collaborative manufacturing ecosystems to attract multinational partnerships. These strategic movements are reshaping the competitive dynamics of the small molecule CDMO industry, fostering transparency, reliability, and global standardization.
Market Outlook and Future Opportunities
Between 2025 and 2035, the market will see intensified collaboration between pharmaceutical innovators and contract manufacturers. The rise of virtual pharmaceutical companies, growing emphasis on flexible capacity, and increasing regulatory harmonization across major economies are expected to accelerate global market expansion.
Continuous investments in technology-driven manufacturing and capacity upgrades will position the Small Molecule CDMO Market as a critical pillar supporting the global pharmaceutical supply chain through 2035 and beyond.
Buy Report Now – Click Here to Purchase the Report
Latest Healthcare IT Reports:
- Organ Preservation Solution Market
https://www.futuremarketinsights.com/reports/organ-preservation-solution-market - Digital Pathology Displays Market
https://www.futuremarketinsights.com/reports/digital-pathology-displays-market - Remote ICU Monitoring System Market
https://www.futuremarketinsights.com/reports/remote-icu-monitoring-system-market
Why Choose FMI – Empowering Decisions that Drive Real-World Outcomes:
https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR-certified, Stevie Award recipient, and a member of the Greater New York Chamber of Commerce) provides data-driven market intelligence, advisory, and consulting services across industries such as Packaging, Food & Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals.
With a network of over 400 analysts operating in 110+ countries, FMI delivers research that empowers organizations to make informed strategic decisions, drive sustainable growth, and maintain a competitive advantage in dynamic global markets.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive, Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531 | Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn | Twitter | Blogs | YouTube